BREAKTHROUGH TREATMENT FOR A COMMON FRACTURE
MIDSHAFT CLAVICLE FRACTURES ARE COMMON

LEGACY
TREATMENTS
HAVE BEEN
SUBOPTIMAL
Historically midshaft clavicle fractures have been treated non-operatively or with plates. Both approaches, while appropriate for some cases, have significant drawbacks. Various intramedullary devices have also been tried, but fallen short.
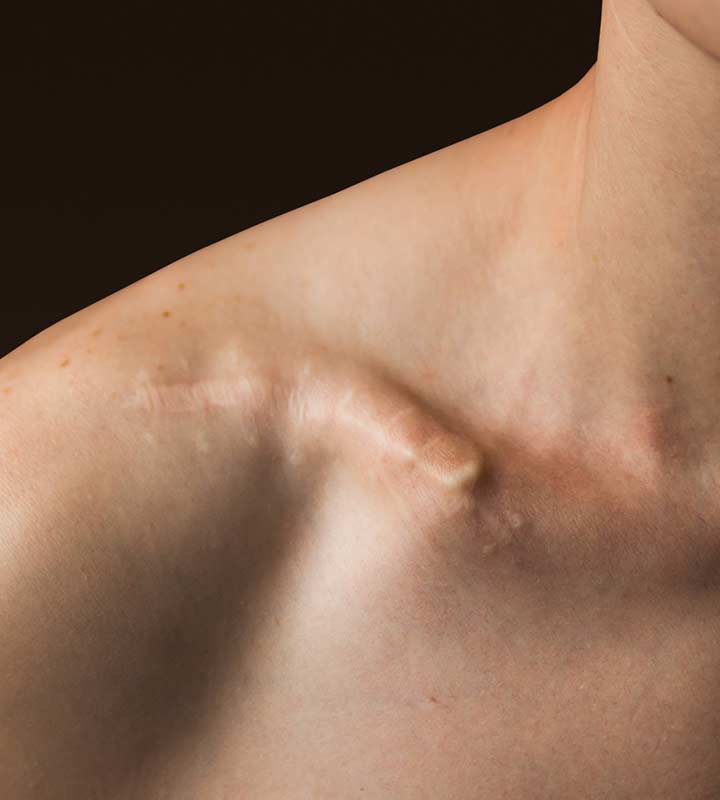
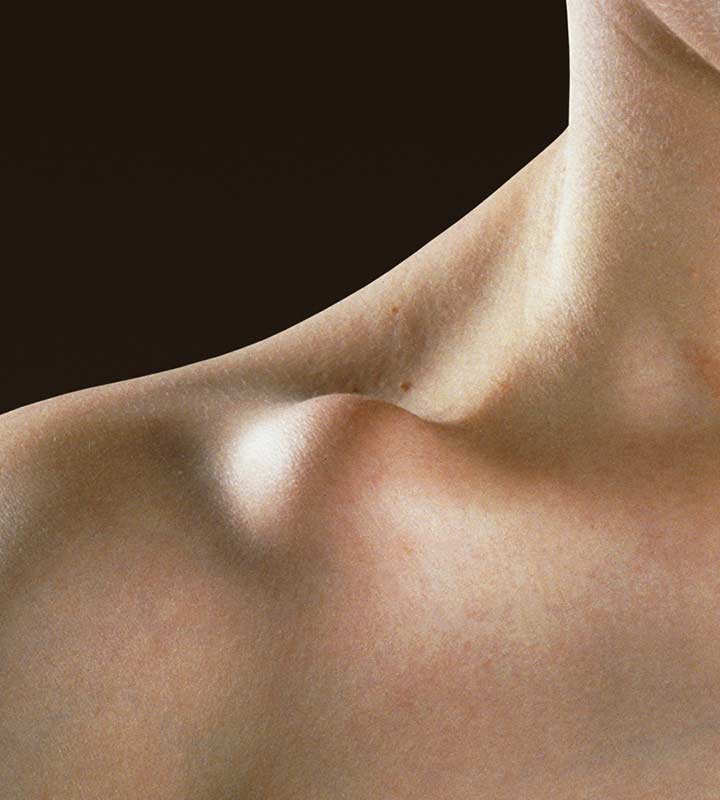
NON-OPERATIVE
– Non-unions, malunions
– Shortening
– Slower recovery
– Initial compromised function
– Poor aesthetics
PLATES
- Large incision
- Supraclavicular neuropathy
- Hardware irritation requiring secondary intervention
- Scarring, poor aesthetics
- Risk of refracture after removal
LEGACY INTRAMEDULLARY DEVICES
- Prominent protruding hardware
- Telescoping, migration
- Wound breakdown
- Secondary shortening
- Frequent secondary interventions
- Indicated for a small subset of fractures
THE ANSWER
IS THE ANSER
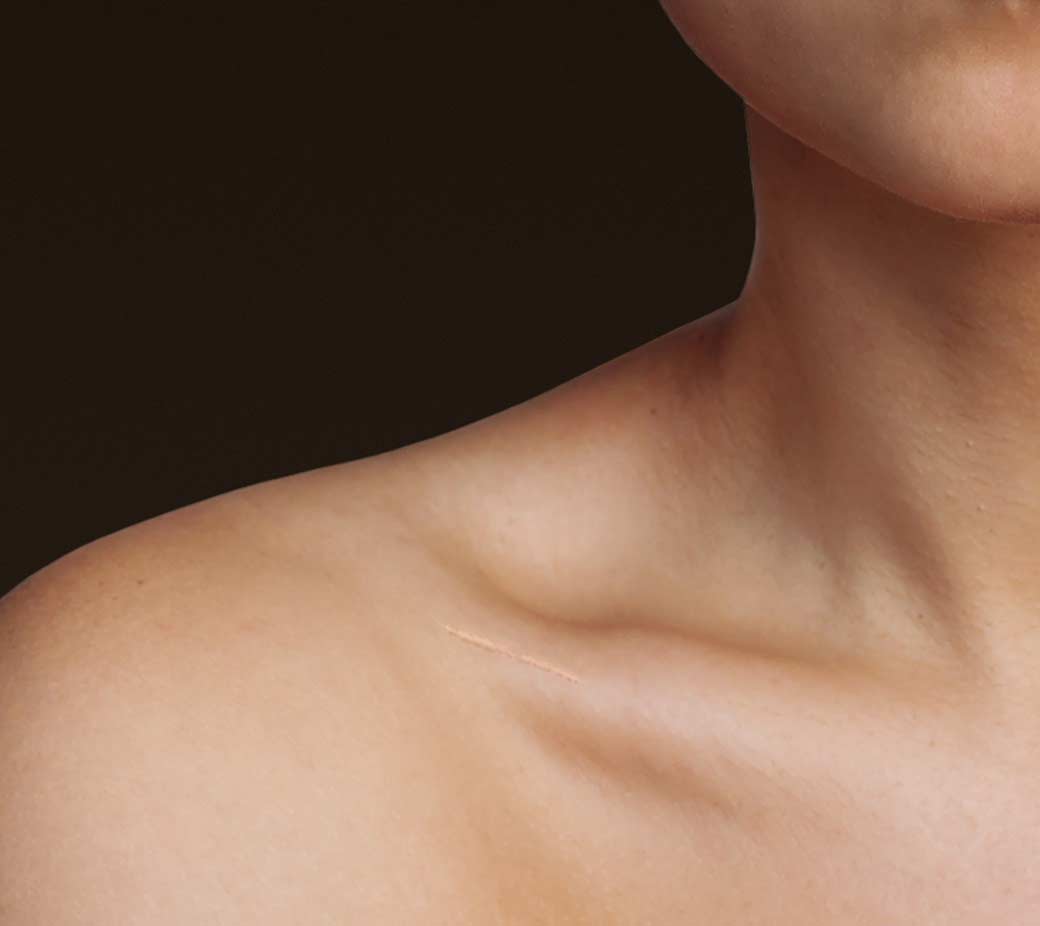
Designed specifically for the surgical management of midshaft clavicle fractures, the Anser Clavicle Pin has the potential to disrupt the market and become the leading treatment in the near future. It solves multiple problems associated with legacy therapies, facilitating excellent clinical and aesthetic outcomes with minimal risk of infection, or need for secondary interventions.
- Minimally invasive procedure
- No bump, no droop, no large scar
- Excellent union rates
- Excellent functional outcomes
- Allows for early rehab, return to activity
- Reduced healthcare cost, time away from work and family
MILESTONES AND FUTURE STATE
Prototype developed in partnership with BAAT Medical BV, Netherlands
Patents granted in USA, Europe, China, Australia, India and South Africa
Multiple Dutch and EU grants obtained
2020 CE certification and 510(k) approval obtained
2023 Introduction at AAOS, US distribution solidified
2024 Dedicated effort to increase US market share
2024 Opening investment round
2025 Global expansion initiatives
2028 Anser Clavicle Pin captures and grows surgical market
POISED FOR SUCCESS
The Anser Clavicle Pin is positioned for global success in the orthopedic and trauma markets. It has been significantly advanced and de-risked, with US and European regulatory approvals, multiple patents, fully validated manufacturing, well-rounded team, strong reimbursement, scalability and scientific data.
Regulatory
Approvals
CE & 510(k)
Enforceable
Patents
AU, SA, IN
Strong
Reimburse-ment
Clinical
Evidence
Peer-reviewed
publications;
Ongoing
prospective trials
Top-tier
Partner
infrastructure,
US & EU
World-class
Advisory Board
Since September 2024, Anser Implants has partnered with Red One Medical for sales in all US Government facilities, including the VA and Military Training Facilities. The establishment of this national contract is critical for our active military personnel, given the demand for an intramedullary clavicle fracture solution.
In their own words, “Red One Medical is a Service-Disabled Veteran Owned Small Business (SDVOSB) supporting America’s warfighters, Veterans, and their families. We focus our efforts on accelerating the transfer of medical technology and acting as a medical innovation hub for the Department of Veterans Affairs (VA) and Department of Defense Military Health System (MHS). Red One works with large companies as well as innovative startups to provide the MHS and VA with groundbreaking medical devices, pharmaceuticals, biologics, and other products that improve patient care.”

